Answer:
The concentration of the acid is 0.05M.
Step-by-step explanation:
1st) The balanced chemical equation of this reaction is:

Here 1 mole of NaOH reacts with 1 mole of HCl.
2nd) With the volume (50.0mL) and the concentration (0.1M) of the NaOH solution, we can calculate the number of moles of NaOH that reacted with the HCl:
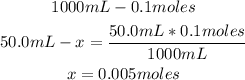
Now we know that 0.005 moles of NaOH reacted to neutralize the 100mL of HCl.
3rd) Since the relation between the reactants in the balanced chemical equation is 1:1, the 0.005 moles of NaOH reacted with 0.005 moles of HCl.
Now, knowing the moles of HCl and the volume of the solution (100mL), we can calculate the concentration of the acid:
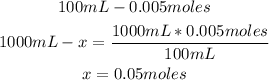
So, the concentration of the acid is 0.05M.