Answer : The correct option is, 2.123 V
Explanation : Given,
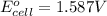
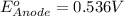
The overall balanced equation of the cell is,

To calculate the
 of the reaction, we use the equation:
of the reaction, we use the equation:
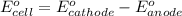
Putting values in above equation, we get:
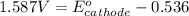
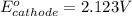
Hence, the
 redox of cathode half cell is, 2.123 V
redox of cathode half cell is, 2.123 V