Answer : The energy of a photon is,

Explanation :
Formula used :

where,
E = energy = ?
h = Planck's constant =
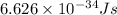
c = speed of light =
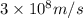
 = wavelength =
= wavelength =

Now put all the given values in the above formula, we get the energy.


Therefore, the energy of a photon is,
