Answer
84.8485 g
Step-by-step explanation
Initial temperature, T₁ = 148 °C
Final temperature, T₂ = 20.4 °C
ΔT = T₂ - T₁
ΔT = 20.4 - 148
ΔT = -127.6 °C
Since heat is released, i.e heat loss, then Q = -324.8 calories
specific heat of the lead bar, c = 0.0300 cal/g°C
Using Q = mcΔT, we can find m as shown below
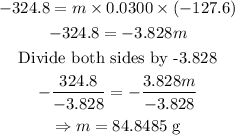
The mass of the bar is 84.8485 g