Answer : The mole ratio of hydrogen to ammonia is, 3 : 2
Explanation :
Balanced chemical reaction : The chemical reaction in which the number of individual atoms of an element present on the reactant side always be equal to the number of individual atoms of an element present on the product side.
The given balanced chemical reaction is,
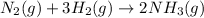
From the balanced chemical reaction, we conclude that 1 mole of
 gas react with 3 moles of
gas react with 3 moles of
 gas to give 2 moles of
gas to give 2 moles of
 gas as a product.
gas as a product.
The mole ratio of nitrogen, hydrogen and ammonia is, 1 : 3 : 2
Therefore, the mole ratio of hydrogen to ammonia is, 3 : 2