1) Chemical equation.
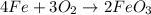
2) Types of reaction.
In the reaction above, we can see that two different compounds get together to form a new compound. This is called a synthesis or a combination.
Decomposition: A compound is broken down into simpler compounds.
Combustion: A compound containing Carbon and Hydrogen combines with oxygen.
Double replacement: Positive and negative ions in two compounds switch places.