Answer : The percent yield of
 is, 45.97 %
is, 45.97 %
Explanation :
First we have to calculate the moles of water.
The balanced chemical reaction is,

From the balanced reaction, we conclude that
As, 1 moles of
 react to give 2 moles of
react to give 2 moles of

So, 2 moles of
 react to give
react to give
 moles of
moles of

Now we have to calculate the mass of
 .
.
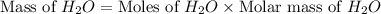
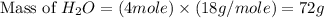
The mass water produces, 72 g
Now we have to calculate the percent yield of
 .
.
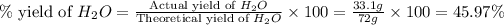
Therefore, the percent yield of
 is, 45.97 %
is, 45.97 %