Answer
When solid iodine is heated, the following equilibrium is set up;
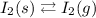
At phase equilibrium, the rate of the forward reaction and the rate of reverse reaction becomes equal. This implies that the concentration of the solid iodine and iodine vapour becomes fairly stable because the rate at which the solid is converted to vapour equals the rate at which the vapour is converted to solid at phase equilibrium.