Step-by-step explanation
Given
Mass of SO2 = 26.2 g
Mass of H2O = 7.4 g
Required: Mass of H2SO3
Solution
Step 1: write a balanced chemical equation
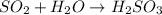
Step 2: Calculate the moles of H2SO3 to be produced by SO2
n = m/M where n is the moles, m is the mass and M is the molar mass
n = 26.2g/64,066 g/mol
n = 0.4089 mol
molar ratio between SO2 and H2SO3 is 1:1 there moles of H2SO3 = 0.4089 mol
Step 3: Calculate the moles of H2SO3 to be produced by H2O
n = m/M where n is the moles, m is the mass and M is the molar mass
n = 7.4g/18,01528 g/mol
n = 0.4108 mol
molar ratio between H2O and H2SO3 is 1:1 there moles of H2SO3 = 0.4108 mol
Therefore the limiting reagent is SO2 because it has less number of moles.
Step 4: Calculate the mass of H2SO3
m = n x M
m = 0.4089 x 82,07 g/mol
m = 33.56 g
Answer
mass of H2SO3 = 33.56 g