Answer:
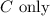
Step-by-step explanation:
Here, we want to get the mass ratio
Firstly, we need to understand the law of definite proportion
What the law basically states is that the components of a molecule are present in a fixed ratio, no matter the source
So, irrespective of the mass, the atoms are expected to be in a definite ratio
Thus, the correct answer here is that:
1.5g of SO2 molecules contain the same mass ratio of S to O atoms as 3.0 g of SO2