Answer
49.0 grams of oxygen is needed.
Step-by-step explanation
Given:
Mass of Aluminum = 55.1 g
What to find:
The mass of oxygen needed to react with the Al.
Step-by-step solution:
The equation for the reaction is:
4Al + 3O₂ → 2Al₂O₃
From the equation; 4 moles of Al reacts with 3 moles of O₂
1 mole of Al = 26.982 g/mol
1 mole of O₂ = 31.998 g/mol
It implies;
(4 mol x 26.982 g/mol) = 107.928 g of Al reacts with (3 mol x 31.998 g/mol) = 95.994 g of O₂
So, 55.1 g of Al will need
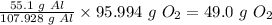
Therefore, the mass of oxygen needed to react with 55.1g of aluminum in the synthesis of aluminum oxide is 49.0 grams