The important relationship or constant we have to know here is the Avogadro's number. 6.022*10^23 /mole. To answer this, we have to convert the mass of Calcium to moles by dividing it with its molar mass. The molar mass of Calcium is 40 g/mol.
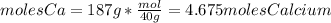
Then we multiply the moles calcium with the Avogadro's constant.
4.675 moles Calcium *
6.022*10^23 /mole = 2.82*10^24 atoms of calcium.