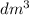moles of HCl = (molarity) * (volume)
=

= 0.02 mol
mole ratio of NaOH : HCl according to the equation is 1 : 1
∴ mol of NaOH = mol of HCl
= 0.02mol
Since volume of NaOH is 0.010
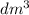
then molarity of NaOH =
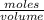
=
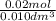
=
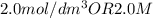
Note:
1)
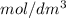
is the same as M
2) I converted the ml to l then to