Answer:
The mass of the ring is 6.3 kg.
Step-by-step explanation:
It is given that,
Density of platinum,

A platinum ring is placed in a graduated cylinder that contains water. the water level rises from 5.0 ml to 5.3 ml when the ring is added. the volume of the ring is, (5.3 - 5) mL = 0.3 mL
Volume of the ring,

We need to find the mass of the ring. The density of any substance is given by :
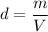


m = 6.3 kg
So, the mass of the ring is 6.3 kg. Hence, this is the required solution.