1) Hypothetical chemical equation
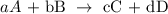
2) The equilibrium constant expression is
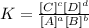
3) Option D. The equilibrium law constant is equal to the product of the equilibrium concentrations of the products divided by the product of the equilibrium concentrations of the reactants raised to the power of their respective coefficients in the balanced equation.