Answer:
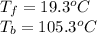
Step-by-step explanation:
Hello,
In this case, it firstly necessary to understand how the complete (100%) dissociation of the potassium phosphate is done:
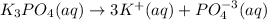
In such a way, since the freezing point depression and the boiling point elevation are computed as:
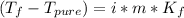
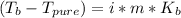
The corresponding van't Hoff factor is four as 3 potassiums and 1 phosphate as ions are obtained during the dissolution. In addition, the freezing point depression constant and boiling point elevation constant of water are 1.86 and 0.512 °C/m respectively. Moreover, the freezing point of water is 0 °C and the boiling point is 100 °C, therefore, the resulting freezing and boiling temperatures for the 2.59 m solution of potassium phosphate are:
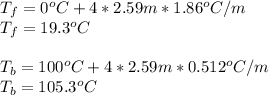
Best regards.