The molarity is calculated using the following formula:
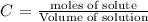
We need to first find the number of moles of CuSO4. The mass = 35.00g and molar mass = (63.55 + 32.07 + (16x4) = 159.62 g/mol. Volume of the solution = 250.0 mL = 0.250 L
n = m/M
n = 35.00 g/159.62 g/mol
n = 0.219 mol
C = 0.219 mol/0.250 L
C = 0.8771 mol/L
Therefore the answer is B.