Answer : The amount of mass in kilograms lost would be,

Solution : Given,
Energy of released in the reaction =

Speed of light = c =
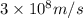
Formula used :

where,
E = energy released
m = mass
c = speed of light
Now put all the given values in the above formula, we get the amount of mass would have been lost.


conversion :

Therefore, the amount of mass in kilograms lost would be,
