In this problem, Charles' law is applicable since constant pressure with varying volume and temperature are given. Charles' law derived from ideal gas law is expressed as
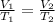
. Subsituting V1=556 cm3, T1=278 K and T2=231 K, V2 or the new volume is 462 cm3.