Answer : Heat required, Q = 5000 cal
Explanation :
It is given that,
Specific heat of water, c = 1 calorie/gram °C
Mass of water, m = 500 grams
Change in temperature,
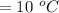
We have to found the amount of heat required. Mathematically, it can be written as :

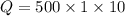
Q = 5000 cal
So, the correct option is (B) " 5000 cal ".
Hence, this is the required solution.