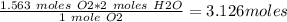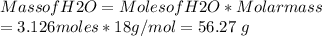Answer:
Mass of H2O = 56.27 g
Step-by-step explanation:
Given:
Molar mass of H2O = 18.00 g/mol
Molar mass of O2 = 32.00 g/mol
Mass of O2 produced = 50.00 g
To determine:
Mass of H2O reacted
Step-by-step explanation:
Water breaks apart during electrolysis to produce O2 and H2. This can be represented as:
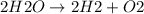
Moles of O2 produced =
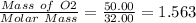
Based on the reaction stoichiometry:
2 moles of H2O produces 1 mole of O2
Therefore, moles of H2O that would react to produce 1.563 moles of O2 is:
=