Answer : The correct option is, 1.0 mole
Explanation : Given,
Molarity of solution = 2.0 mole/L
Volume of solution = 0.50 L
Molarity : It is defined as the number of moles of solute present in one liter of solution.
Formula used :
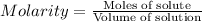
Now put all the given values in the above formula, we get the moles of the solute.
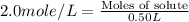
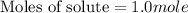
Therefore, the number of moles of solute dissolved in the solution will be, 1.0 mole