Answer:
The stoichiometric coefficients.
Step-by-step explanation:
Hello,
Chemical reactions are widely generalized by the following chemical equation:
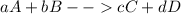
Capitalized letters are referred to both the reacting and produced species during the chemical reaction meanwhile the lowered case letters are referred to the stoichiometric coefficients which balance the chemical reaction. Balancing means that the number of atoms in the reagents must be equal to the number of atoms in the products, this is exemplified via the following balanced chemical reaction:

The coefficient 2 in the hydrochloric acid is set due to the produced calcium chloride which has two chlorine atoms in order to equal them.
Best regards.