Answer: The heat required by aluminium will be 10800 J
Step-by-step explanation:
To calculate the amount of heat absorbed or released, we use the equation:

where,
q = amount of heat absorbed or released = ? J
m = mass of the substance = 100 g
c = specific heat of aluminium = 0.90 J/ g°C
 = change in temperature =
= change in temperature =
![[150-30]^oC=120^oC](https://img.qammunity.org/2017/formulas/chemistry/high-school/sa7vdn036eir4slo1bzssqo3gd507u6kh6.png)
Putting values in above equation, we get:
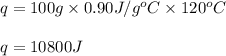
Hence, the heat required by aluminium will be 10800 J