Answer:
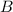
Step-by-step explanation:
Here, we want to get the oxidizing agent in the forward reaction
By definition, the oxidizing agent is the reactant that undergoes reduction
Reduction is simply a gain in electrons. This simply means that the oxidation number decreases or becomes more negative for the substance undergoing reduction
Now, let us look at the reactants:
The nitrogen went from an oxidation number of +3 in NO2- to -3 in NH3
This means it has undergone reduction and it is the oxidizing agent