Answer: Reaction is spontaneous at all temperatures.
Step-by-step explanation:
According to Gibb's equation:

 = Gibbs free energy
= Gibbs free energy
 = enthalpy change
= enthalpy change
 = entropy change
= entropy change
T = temperature in Kelvin
 = +ve, reaction is non spontaneous
= +ve, reaction is non spontaneous
 = -ve, reaction is spontaneous
= -ve, reaction is spontaneous
 = 0, reaction is in equilibrium
= 0, reaction is in equilibrium
Thus when
 = -ve (decrease in enthalpy)
= -ve (decrease in enthalpy)
 = +ve (increase in entropy)
= +ve (increase in entropy)

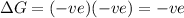
Thus Reaction is spontaneous at all temperatures.