The "Ideal Gas Law Equations" is
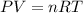
P= Pressure (in Pascals)
V=Volume (in Liters)
n=amount, or
number (in moles)
R= 8.3145
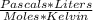
or
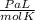
T= Temperature (In Kelvin)
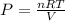
Plug into the equation and you're good!

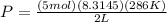
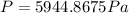
If your teacher cares about sig figs,
2 sig figs (significant figures)
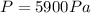
For other units of pressure,
1 atm = 760 mmHG = 760 Torr = 101326 Pa = 1.01325 bar