Answer:
164moles
Step-by-step explanation:
Given parameters:
Number of molecules of CO₂ = 9.86 x 10²⁵molecules
Unknown:
Number of moles = ?
Solution:
To solve this problem, a mole of any substance contains the Avogadro's number of particles.
In that;
6.02 x 10²³ molecules makes up 1 mole of a substance
9.86 x 10²⁵molecules contains
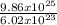 = 1.64 x 10²moles
= 1.64 x 10²moles
The number of moles is 164moles