Answer:
1.33moles
Step-by-step explanation:
Given parameters:
Mass of LiF = 34.58g
Unknown:
Number of moles of LiF = ?
Solution:
To solve this problem, we need to find the molar mass of LiF first.
Molar mass of LiF = 7 + 19 = 26g/mol
Number of moles of LiF =
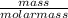
So;
Number of moles of LiF =
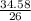 = 1.33moles
= 1.33moles