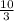Answer:
Step-by-step explanation:
The reaction expression is given as:
CH₂ + O₂ → CO₂ + H₂O
The reaction above needs to be balanced first. Let us take a mathematical approach to solve this;
aCH₂ + bO₂ → cCO₂ + dH₂O
Conserving C: a = c
H: 2a = 2d
O: 2b = 2c + d
let a = 1, c = 1, d = 1 and b =
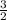
Multiply through by 2;
a = 2, b = 3, c = 2 and d = 2
2CH₂ + 3O₂ → 2CO₂ + 2H₂O
From the balanced reaction expression;
3 moles of O₂ will produce 2 moles of CO₂
5 moles of O₂ will produce
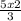 moles of CO₂ =
moles of CO₂ =