Answer:
Energy of photon,

Step-by-step explanation:
It is given that,
Wavelength of photon,

It strikes a mercury atom in the ground state. We have to find the energy of this photon. It can be calculated using below relation as :
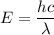
Where
h is the Planck's constant
c is the speed of light
So,


Hence, the above value is the energy of this photon.