Answer: The correct answer is Option 1.
Step-by-step explanation:
It is given that the process is endothermic which means that

The snow is subliming which means that it is changing its state from solid state to gaseous state directly and for this phase change to take place,
 must be negative.
must be negative.
As, for sublimation process,
 is always increasing and for spontaneous reaction, temperature must also be high.
is always increasing and for spontaneous reaction, temperature must also be high.

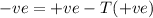
At higher temperature,
 will always be negative and it will be spontaneous reaction and phase change will occur.
will always be negative and it will be spontaneous reaction and phase change will occur.
Hence, the correct answer is Option 1.