Oxidation State of Fe in Iron (III) Oxide:
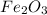
let oxidation state of Fe = x
2x + (-2 * 3) = 0
2x - 6 = 0
2x = +6
x = +3
Oxidation state of Solid Iron
Fe
the rule is that the oxidation state of an uncombined element is zero
Redox pertains to electron transfer and not proton transfer thus your answer is
OPTION 3 (+3 to 0 as electrons are transferred)