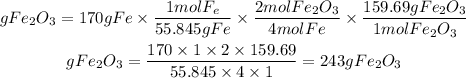Step-by-step explanation
We are given the balanced equation for the reaction, so we can continue with the calculations.
To solve the question we will follow the following steps:
1. We calculate the moles of Fe present in 170g. For these, we use the atomic mass of Fe equal to 55.845 g/mol.
2. By stoichiometry we find the moles of Fe2O3 that will be produced. We have that for every 4 moles of Fe, 2 moles of Fe2O3 are obtained. Therefore, the ratio Fe2O3 to Fe is 2/4=1/2.
3. We calculate the grams of Fe2O3 by multiplying the moles by the molar mass of Fe2O3 equal to 159.69g/mol
Let's proceed with the calculations
1. Moles of Fe
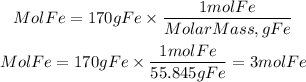
2. Moles of Fe2O3
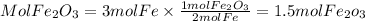
3. Grams of Fe2O3
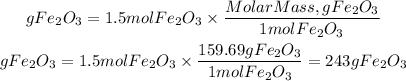
Answer: If we start with 170 g Fe will be produced 243g Fe2O3
Student correction
Highlighted are the numbers that are incorrect. Remember that when we use the relationship for molar mass, the weight we have is only for one mole of the compound. Therefore, you should not put any number other than 1 when you put the molar mass. So following your nomenclature the equation would be: