The molarity or molar concentration of a solution is given by the number of moles of solute divided by the volume of solution:
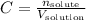
No solute was added when the solution was diluted, so the only terms that change are the concentration and the volume.
So, let 1 denote the situation before the dilution and 2 denote the situation after the dilution.
At first, we have concentration 1, volume 1 and number of moles 1:
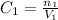
After diluting, we have concentration 2, volume 2 and number of moles 2:
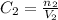
However, since no solute was added or removed, the number of moles before and after are the same:
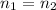
So, if we solve both equations for n, we have:
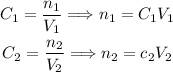
So:
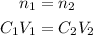
The initial concentration and volume are:
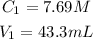
And it was diluted to 137.6 mL, so this is the final volume:
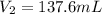
Solving the equation we have to C2, we have:
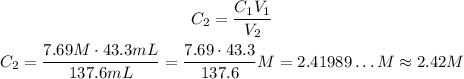
So, the resulting molarity is approximately 2.42 M.