We are given the molarity of the solution, the molarity corresponds to the following expression:
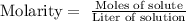
That is, in one liter of solution there will be 0.259 moles of calcium sulfate.
We are asked to find the volume to have 4.45g, let's see how many moles are equivalent. To do this we divide the required weight by the molecular weight (MW) of calcium sulfate.
MW of calcium sulfate = 136.1 g/mol
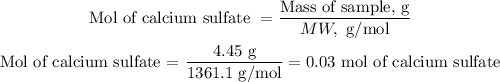
Now we apply a rule of three to know the required volume. In the same operation, we can make the conversion from liters to milliliters, since the answer is asked in milliliters.
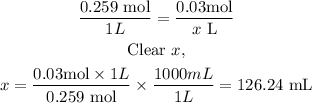
So, we will need 126.24 mL to supply 4.45 g from a 0.259M calcium sulfate solution