1) Determine the mass of zirconium
Convert moles of zircon into moles of zirconium
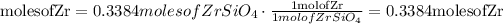
Convert moles of zirconium into mass of zirconium (g)
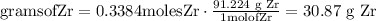
2) Determine the mass of Silicon
Convert moles of zircon into moles of silicon
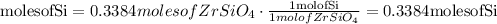
Convert moles of silicon into mass of silicon (g)
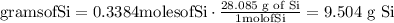
3) Determine the mass of Oxygen
Convert moles of zircon into moles of oxygen
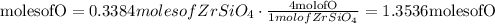
Convert moles of oxygen into mass of oxygen
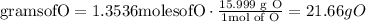
The mass of zirconium is 30.87 g
The mass of silicon is 9.504 g
The mass of oxygen is 21.66 g