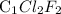
Molecular weight of Freon-12
1 carbon = 12
2 chlorines = 2 x 35 = 70
2 flourines = 19 x 2 = 38
Total mass = 120g
120 g of Freon-12 ---------------------- 12 g are carbon
1200 g of Freon-12 ------------------------ x
x = (1200 x 12) / 120
x = 120 g of Carbon
The answer is 120g