Given data:
The current is I=14.72 A.
The time taken is t=22.94 s.
The charge pass through the wire can be calculated as,
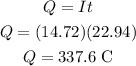
If the charge on an electron is e=1.6x10⁻¹⁹ C, the the number of electrons pass through the wire will be,
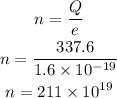
Thus, the number of electrons pass through the wire in 22.94 s is 211x10¹⁹.
Final Answer: 211x10¹⁹