Answer:
The balanced chemical equation is:
![\text{CH}_4\text{ + N}_2\text{Cl}_4\operatorname{\rightarrow}\text{CCl}_4\text{ + 2H}_2\text{ + N}_2]()
Step-by-step explanation:
1st) It is necessary to identify the reactants (on the left side of the arrow) and the products (on the right side of the arrow) following the color reference and theamount of those colors each molecule has:
Reactants:
- CH4 (Methane)
- N2Cl4 (Tetrachlorohydrazine)
Products:
- CCl4 (Carbon tetrachloride)
- H2 (Hydrogen)
- N2 (Nitrogen)
2nd) Now we have to balance the chemical equation making sure there are the same amount of elements on the reactant side as on the product side.
- Unbalanced equation:

- Balanced equation:
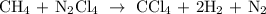
So, the equation is balanced because both sides have:
• 1 C
,
• 4 H
,
• 2 N
,
• 4 Cl