We have to use Avogadro's number to find the number of moles of BaF2. This number says that there are 6.022 x 10^(23) molecules in 1 mol. The calculation would be:
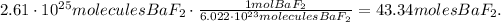
The answer is that we have 43.34 moles of BaF2 in 2.61 x 10^(25) molecules/formula units.