Answer: a)
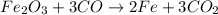
b) 33749996 grams
Step-by-step explanation:
a) The balanced chemical reactions will be :
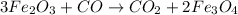
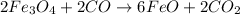
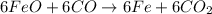
Overall equation :
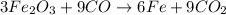
Overall balanced equation :
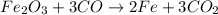
b) Amount of iron = 45 metric ton = 45000 kg = 45000000g
To calculate the moles :
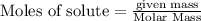
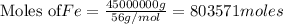
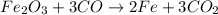
According to stoichiometry :
2 moles of
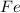 are produced from= 3 moles of
are produced from= 3 moles of
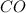
Thus 803571 moles of
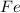 are produced from=
are produced from=
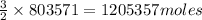 of
of
Mass of
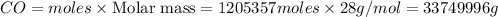
Thus 33749996 g of carbon monoxide are required to form 45.0 metric tons of iron from ferric oxide