Answer:
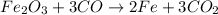 is an oxidation-reduction reaction.
is an oxidation-reduction reaction.
Step-by-step explanation:
In an oxidation-reduction reaction, the oxidation number of some elements must change during reaction.
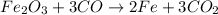 represents an oxidation-reduction reaction.
represents an oxidation-reduction reaction.
oxidation number
Fe in
 +3
+3
Fe in Fe 0
C in CO +2
C in
 +4
+4
SO, oxidation number of Fe decreases during reaction. Hence
 is being reduced. Also, oxidation number of C increases during reaction. Hence CO is being oxidized.
is being reduced. Also, oxidation number of C increases during reaction. Hence CO is being oxidized.
In all other given reactions, oxidation number of elements are not changed during reaction. Hence they are not oxidation-reduction reaction.