Answer
Molarity (C) = 0.41 mol/L
Step-by-step explanation
Given:
Number of moles = 3.5 moles
Volume = 8.50 L
Required: Molarity of the solution
Solution
To solve for molarity, you can use the following equation
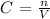
Where C is the concentration or molarity, n is the number of moles and V is the volume of the solution.
C = 3.5 mol/8.50 L
C = 0.41 mol/L