Answer:
76.04mL
Explanations
The formula for calculating the density of a substance is expressed as:
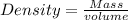
60% m/m = 60 g of ethanol in 100 g of solution
Given that the density of ethanol = 789 kg/m³.
Convert the 60%m/m mass to kg to have;
mass of ethanol required = 0.06kg
Determine the volume
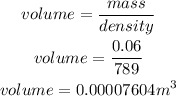
Convert cubic meters to mL
Since 1m³ = 10⁶mL, hence
0.00007604m³ = 0.00007604 * 10⁶
0.00007604m³ = 76.04mL
Hence the volume of ethanol that are in a 100 g of this hand sanitizer is 76.04mL