Answer:
The answer is below
Step-by-step explanation:
Newton's law of gravity states that the force between two bodies is directly proportional to the product of their masses and inversely proportional to the square of the distance between them. The law is expressed by the formula:
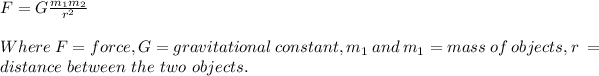
The masses and distances for this question is in common units, Therefore the result would be in ratios
a) 4 MEarth / 2 MSolar / 3 AU
The force (F) = (4 * 3) / 3² = 4/3
b) 1 MEarth / 1 MSolar / 1 AU
The force (F) = (1 * 1) / 1² = 1
c) 1 MEarth / 2 MSolar / 2 AU
The force (F) = (1 * 2) / 2² = 1/2