Answer
1.74 × 10²⁴ molecules are present in 214 g of calcium hydroxide.
Step-by-step explanation
Step 1: Calculate the moles of calcium hydroxide in 214 g using the mole formula.
The molar mass of calcium hydroxide = 74.093 g/mol
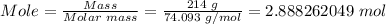
Step 2: Convert 2.888262049 moles of calcium hydroxide to molecules using Avogadro's constant.
Conversion factor: 1 mole of any substance = 6.022 × 10²³ molecules.
∴ 2.888262049 moles of calcium hydroxide is equal to
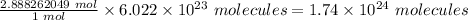
Hence, 1.74 × 10²⁴ molecules are present in 214 g of calcium hydroxide.