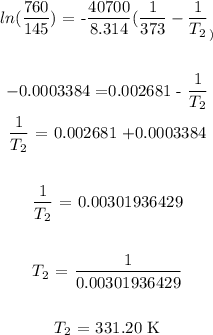
Answer:
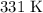
Step-by-step explanation:
Here, we want to get the vapor pressure
We can find this by using Clausius-Clapeyron equation
Mathematically, we have it that:
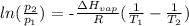
where:
P2 equals final pressure which is temperature at boiling point which is 760 torr
P1 is 145 torr
Heat of vaporization is 40,700 Kj/mol
R is molar gas constant which is 8.314
T1 is initial temperature which is 373 K (the boiling point of water)
T2 is final temperature which is what we want to calculate
Substituting the values, we have it that: