16. b) This question is asking for the enthalpy change for the following reaction:
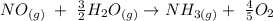
Note that the reaction is reversed meaning the enthalpy will be = 906 kJ
Also note that the reaction was divided by 4, therefore
DH = 906/4
DH = 226.5 kJ
16. c) This question is asking us to calculate the energy that will be given off if 14.3g NH3 is burned in the presence of O2.
The first thing to do is to make sure the equation is balanced.
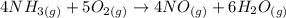
In this reaction, 4 moles of NH3 reacts with 5 moles of oxygen and 4 moles of NO and 6 moles of water are produced, and the enthaply is -906kJ. Therefore if 4 moles of NH3 reacts, 906 kJ of heat will be given off, that is why there is a minus sign for enthalpy.
To get the energy given off by 14.3g NH3, we will first calculate the number of moles of NH3.
n=m/M where m is the mass and M is the molar mass of NH3.
n=14.3g/17.031g/mol
n=0.84 mol
Now that we do have number of moles, we can use the known enthalpy change.
When 4 moles react, 906 kJ is given off.
Energy given off = 0.82 moles of NH3 x (906 kJ/4 moles of NH3)
Energy given off (DH) = 190.18 kJ
.