Answer : The number of moles present in the oxygen gas is, 272.625 moles and the pressure of oxygen gas in atmosphere in the cylinder is, 264.56 atm
Solution : Given,
Mass of oxygen gas = 4.362 Kg = 4362 g (1 Kg = 1000 g)
Molar mass of oxygen gas = 16 g/mole
First we have to calculate the moles of oxygen gas.
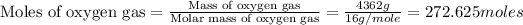
Now we have to calculate the pressure of oxygen gas by using ideal gas equation.
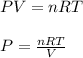
where,
P = pressure of gas
T = temperature of gas =
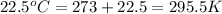
V = volume of gas = 25 L
n = number of moles of gas = 272.625 moles
R = gas constant = 0.0821 Latm/moleK
Now put all the given values in the ideal gas equation, we get the pressure of gas.
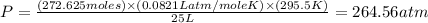
Therefore, the number of moles present in the oxygen gas is, 272.625 moles and the pressure of oxygen gas in atmosphere in the cylinder is, 264.56 atm